ICLR, ‘23
DiffDock: Diffusion Steps, Twists, and Turns for Molecular Docking
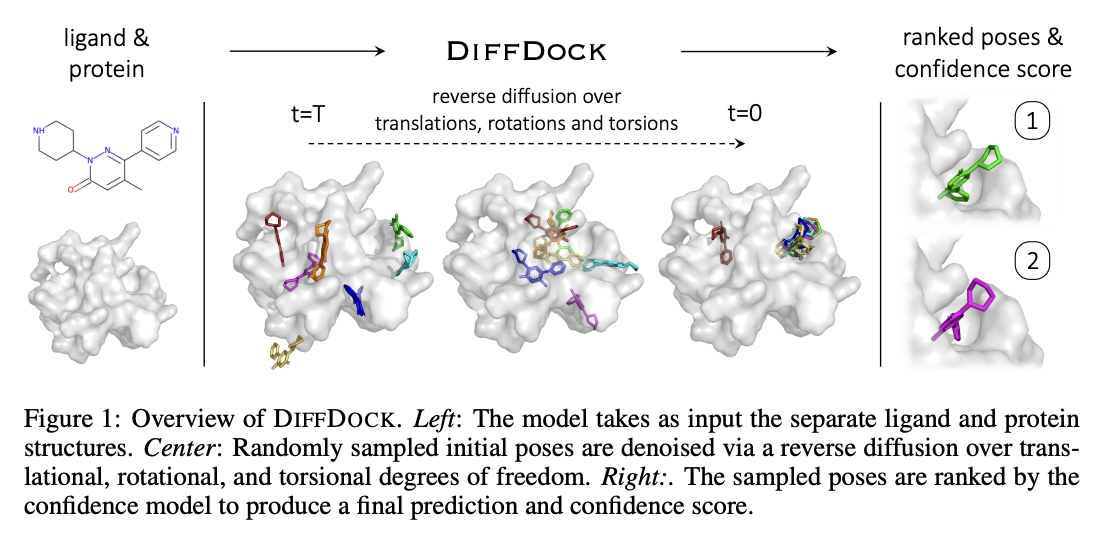
-
This article is one of the first research work that formulated molecular docking as a generative problem.
-
Showed very interesting results with decent performance gain.
-
If you are interested in molecular docking and diffusion models, this is definitely a must-read paper!
-
It is highly recommended to watch youtube video explained by the authors.
Summary
-
Molecular docking as a generative problem, not regression!
- Problem of learning a distribution over ligand poses conditioned on the target protein structure $p(\mathbf{x} | \mathbf{y})$
-
Used “Diffusion process” for generation
-
Two separate model
-
Score model: $s(\mathbf{x}, \mathbf{y}, t)$
Predicts score based on ligand pose $\mathbf{x}$, protein structure $\mathbf{y}$, and timestep $t$
-
Confidence model: $d(\mathbf{x}, \mathbf{y})$
Predicts whether the ligand pose has RMSD below 2Å compared to ground truth ligand pose
-
-
Diffusion on Product space $\mathbb{P}$
- Reduced degrees of freedom $3n \rightarrow (m+6)$
Preliminaries
Molecular Docking
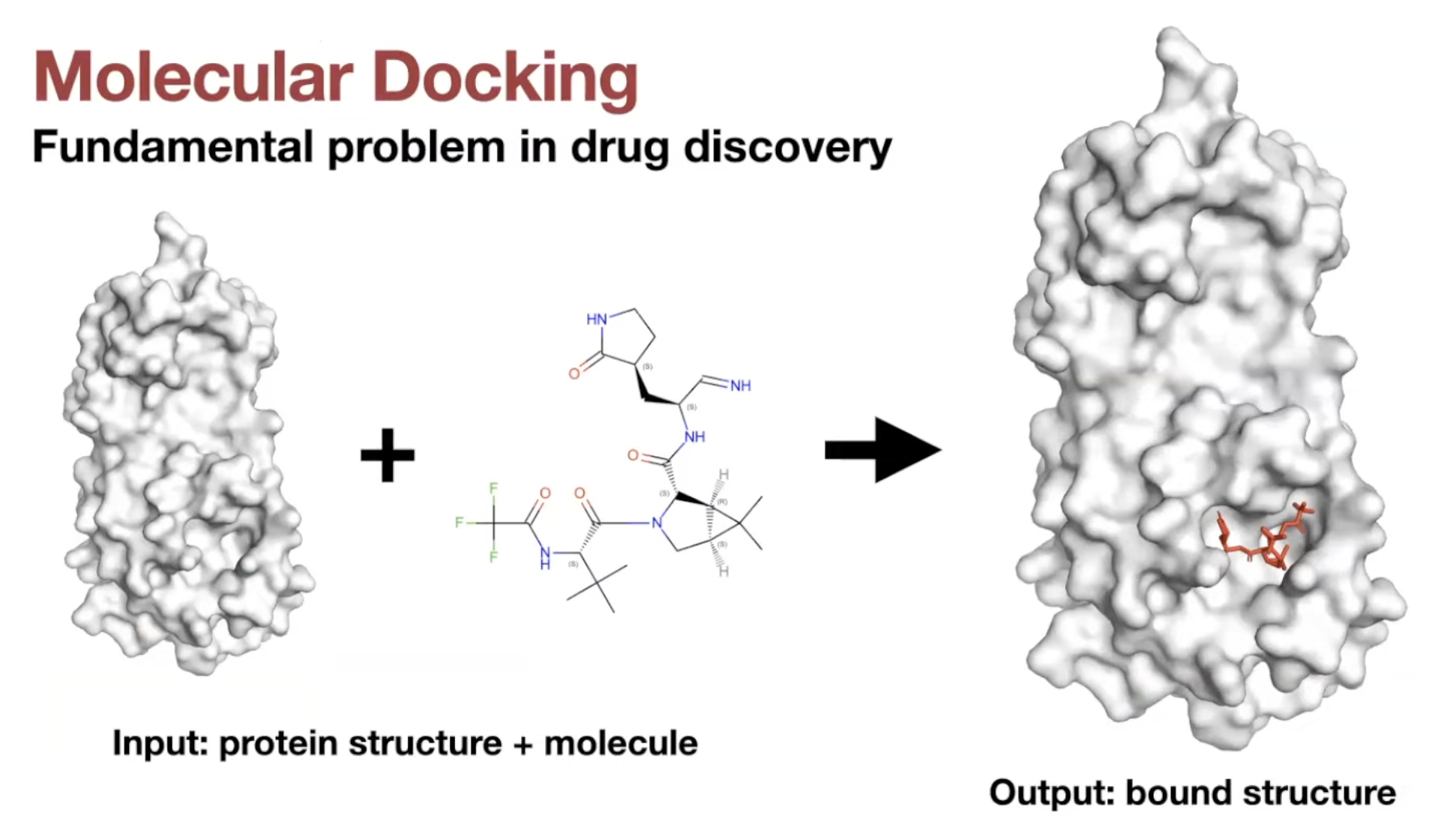
-
Definition:
Predicting the position, orientation, and conformation of a ligand when bound to a target protein
-
Two types of tasks
- Known-pocket docking
- Given: position of the binding pocket
- Blind docking
- More general setting: no prior knowledge about binding pocket
- Known-pocket docking
Previous works: Search-based / Regression-based
-
Search based docking methods
-
Traditional methods
-
Consist of parameterized physics-based scoring function and a search algorithm
-
Scoring function
- Input: 3D structures
- Output: estimate of the quality/likelihood of the given pose
-
Search algorithm
- Stochastically modifies the ligand pose (position, orientation, torsion angles)
- Goal: finding the global optimum of the scoring function.
-
ML has been applied to parameterize the scoring function.
- But very computationally expensive (large search space)
-
Example

-
-
Regression based methods
-
Recent deep learning method
-
Significant speedup compared to search based methods
-
No improvements in accuracy
-
Example

-
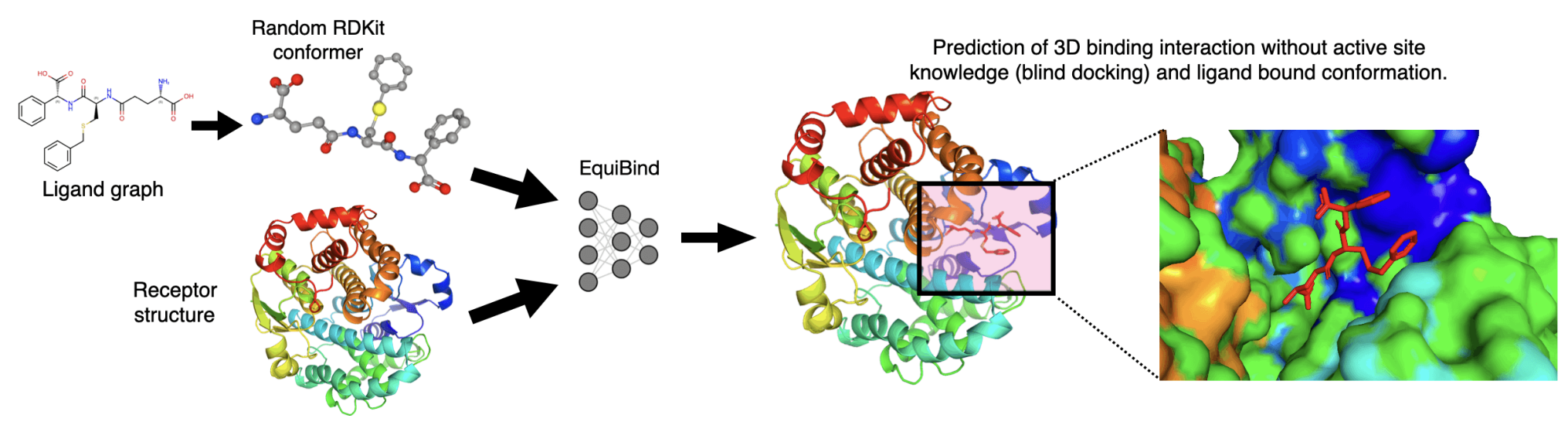
- Tried to tackle the blind docking task as a regression problem by directly predicting pocket keypoints on both ligand and protein and aligning them.
-
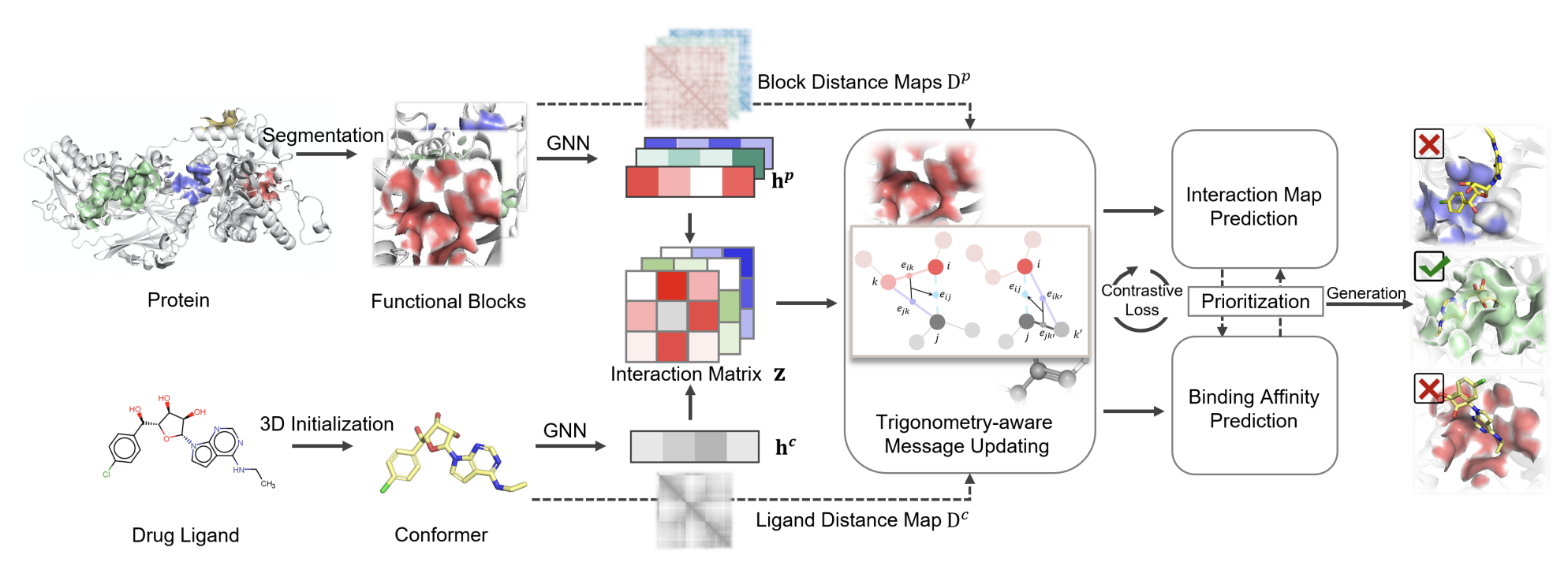
- Improved over this by independently predicting a docking pose for each possible pocket and then ranking them.
-
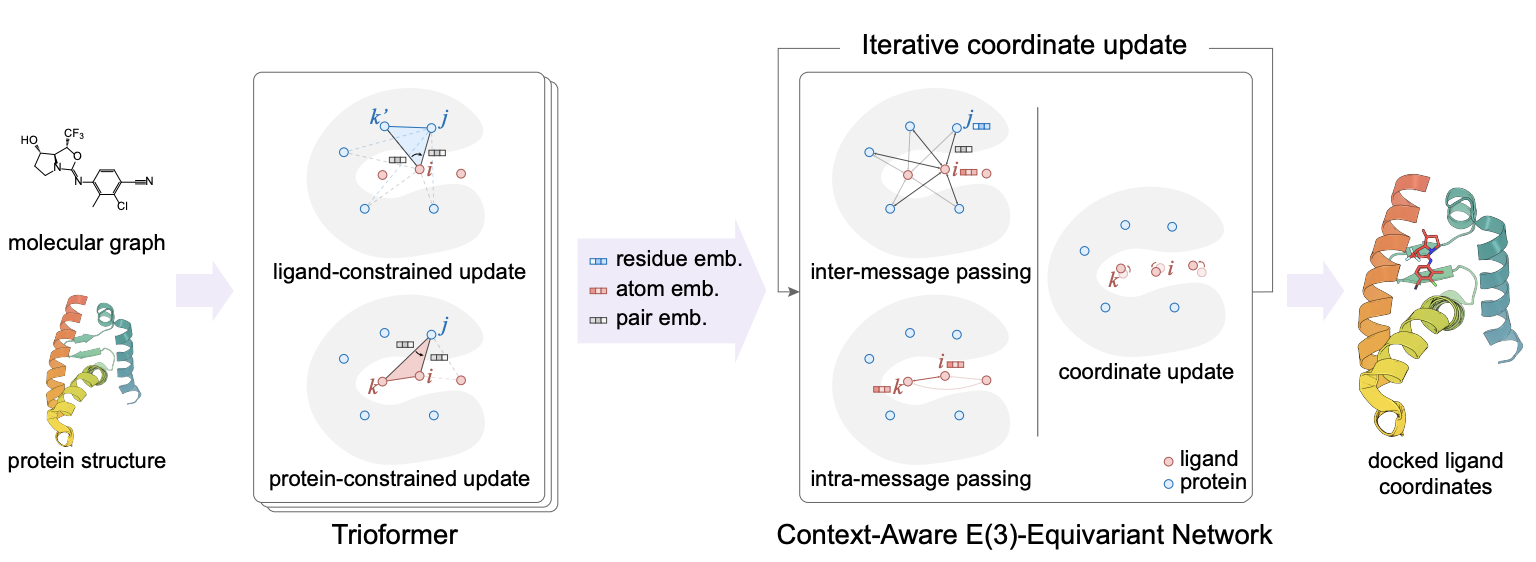
- Used ligand-constrained & protein-constrained update layer to embed ligand atoms and iteratively updated coordinates.
-
-
Docking objective
- Standard evaluation metric:
-
$\mathcal{L}_{\epsilon} = \sum_{x, y} I_{\text{RMSD}(y, \hat{y}(x))<\epsilon}$:
proportion of predictions with $\text{RMSD} < \epsilon$ → Not differentiable!
-
Instead, we use $\text{argmin}_{\hat{y}} \lim_{\epsilon \rightarrow 0} \mathcal{L}_\epsilon$ as objective function.
-
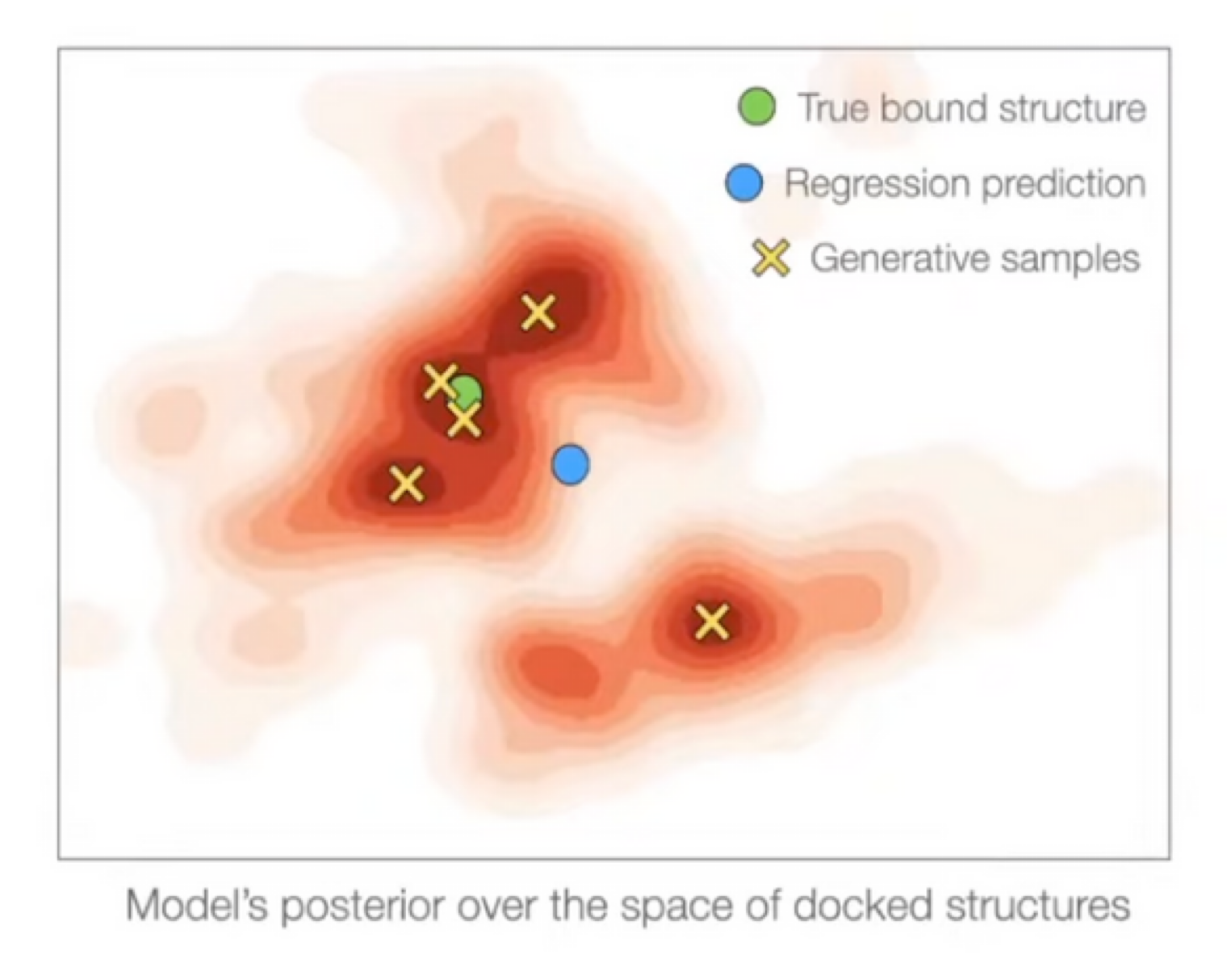
- Regression is suitable for docking only if it is unimodal.
- Docking has significant aleatoric (irreducible) & epistemic (reducible) uncertainty
- Regression methods will minimize $\sum \|y - \hat{y}\|^2_2$ → will produce weighted mean of multiple modes
- On the other hand, generative model will populate all/most modes!
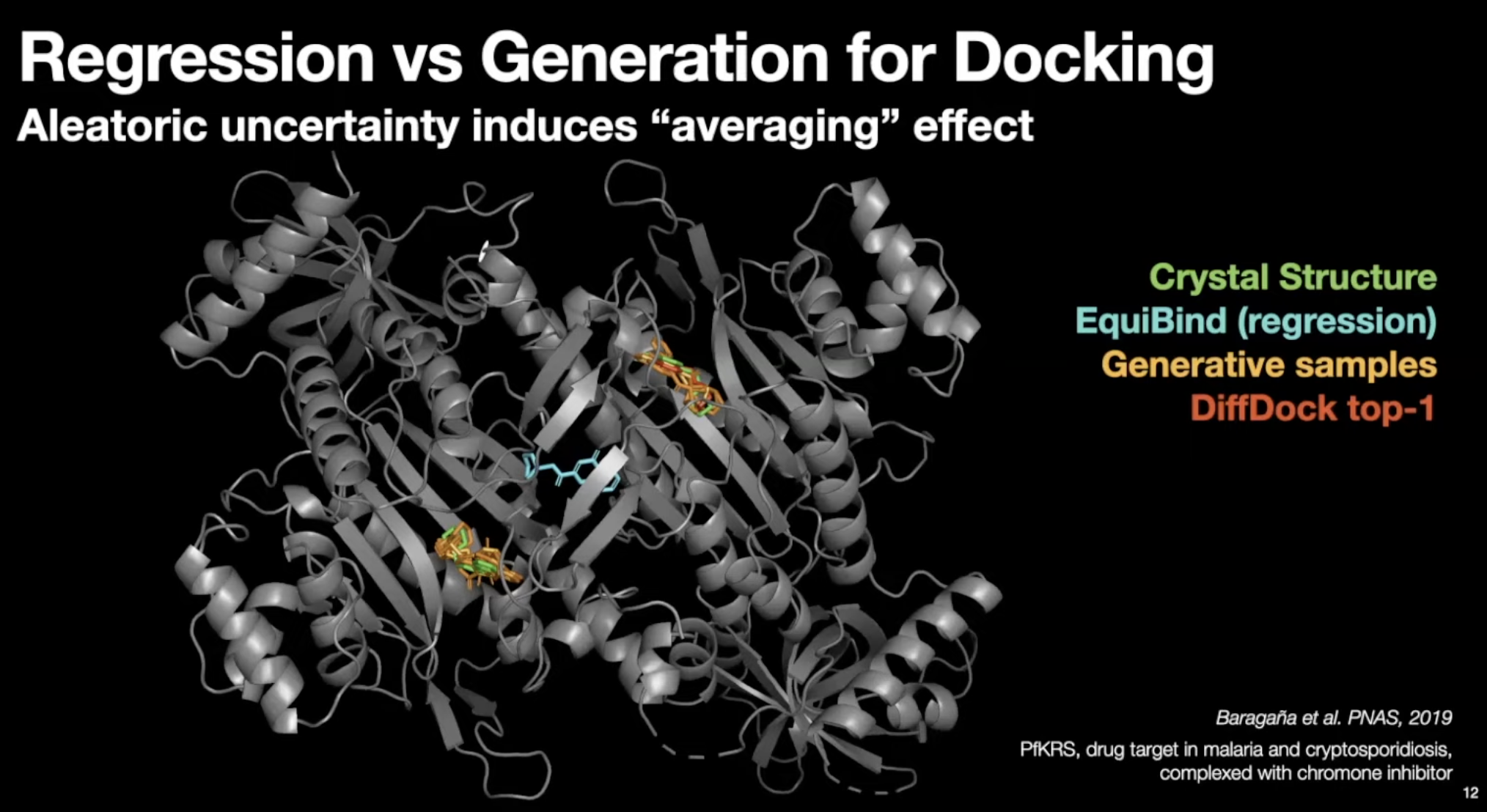
- Regression (EquiBind) model set conformer in the middle of the modes.
- Generative samples can populate conformer in most modes.
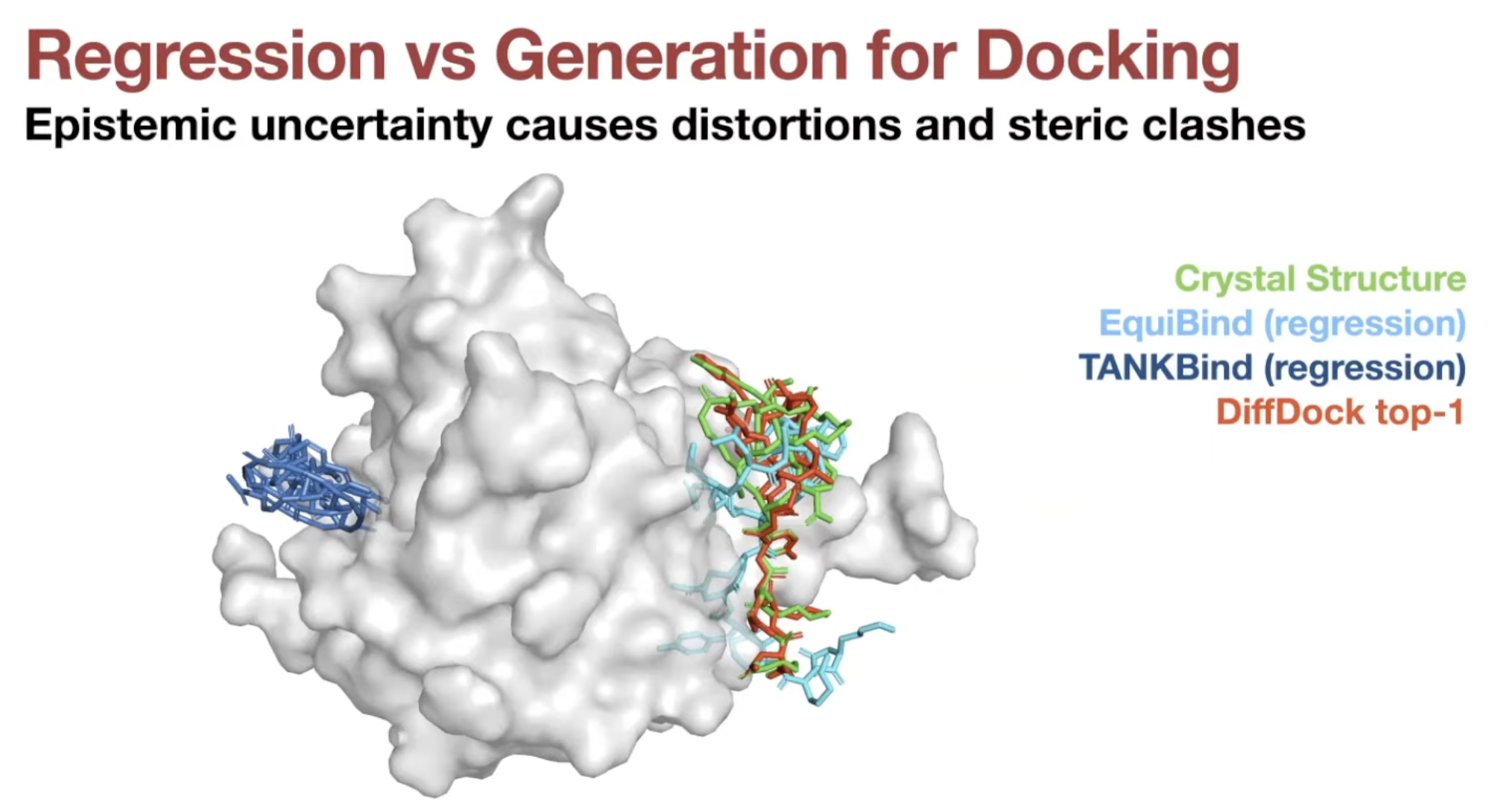
- Much less steric clashes for generative models
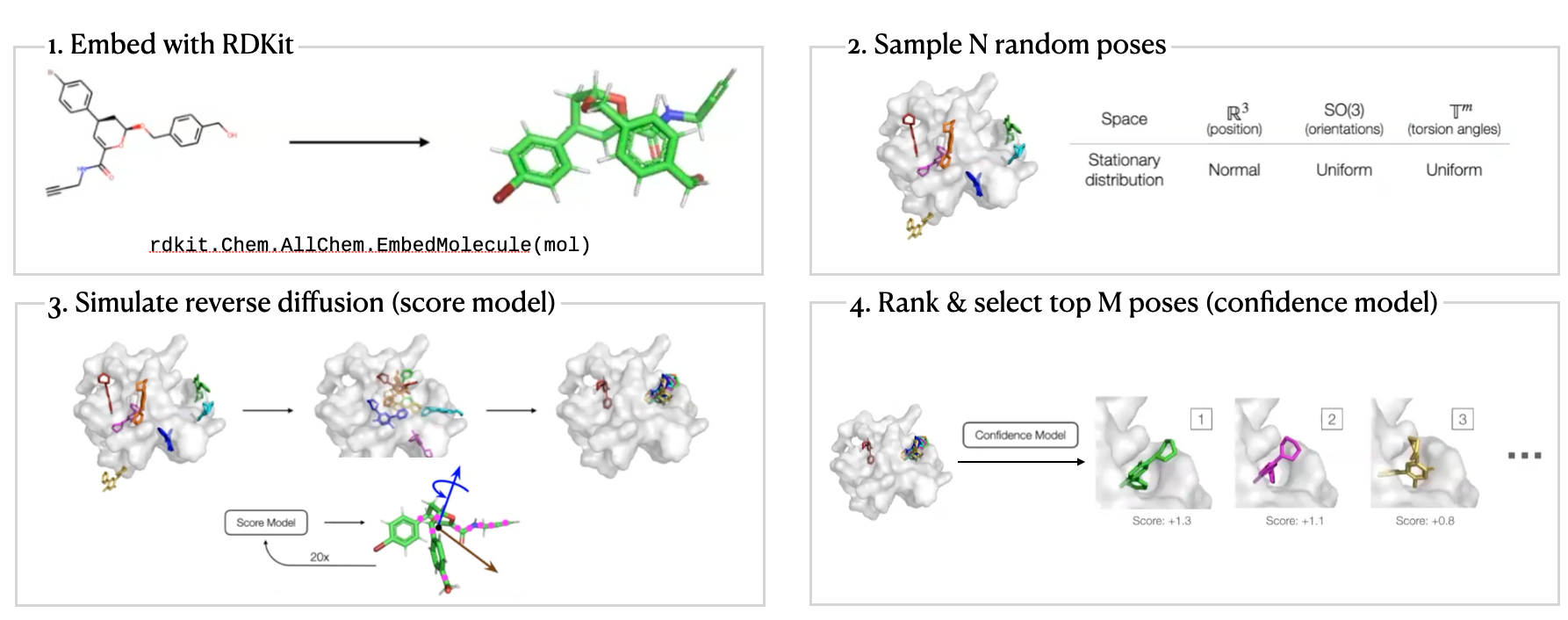
DiffDock Overview
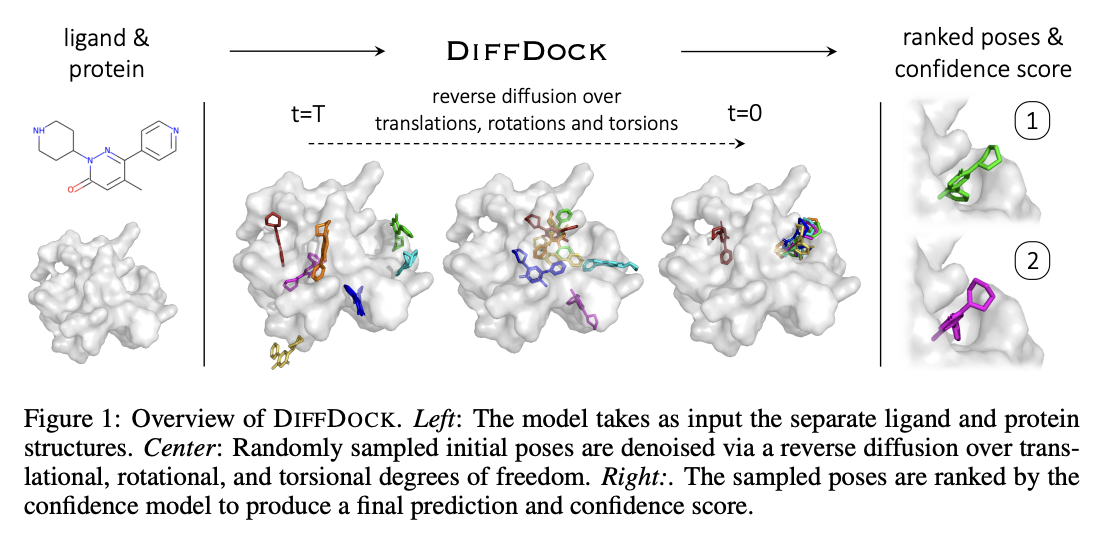
- Two-step approach
- Score model: Reverse diffusion over translation, rotation, and torsion
- Confidence model: Predict whether or not each ligand pose is $\text{RMSD} < 2\text{Å}$ compared to ground truth ligand pose
Score model
-
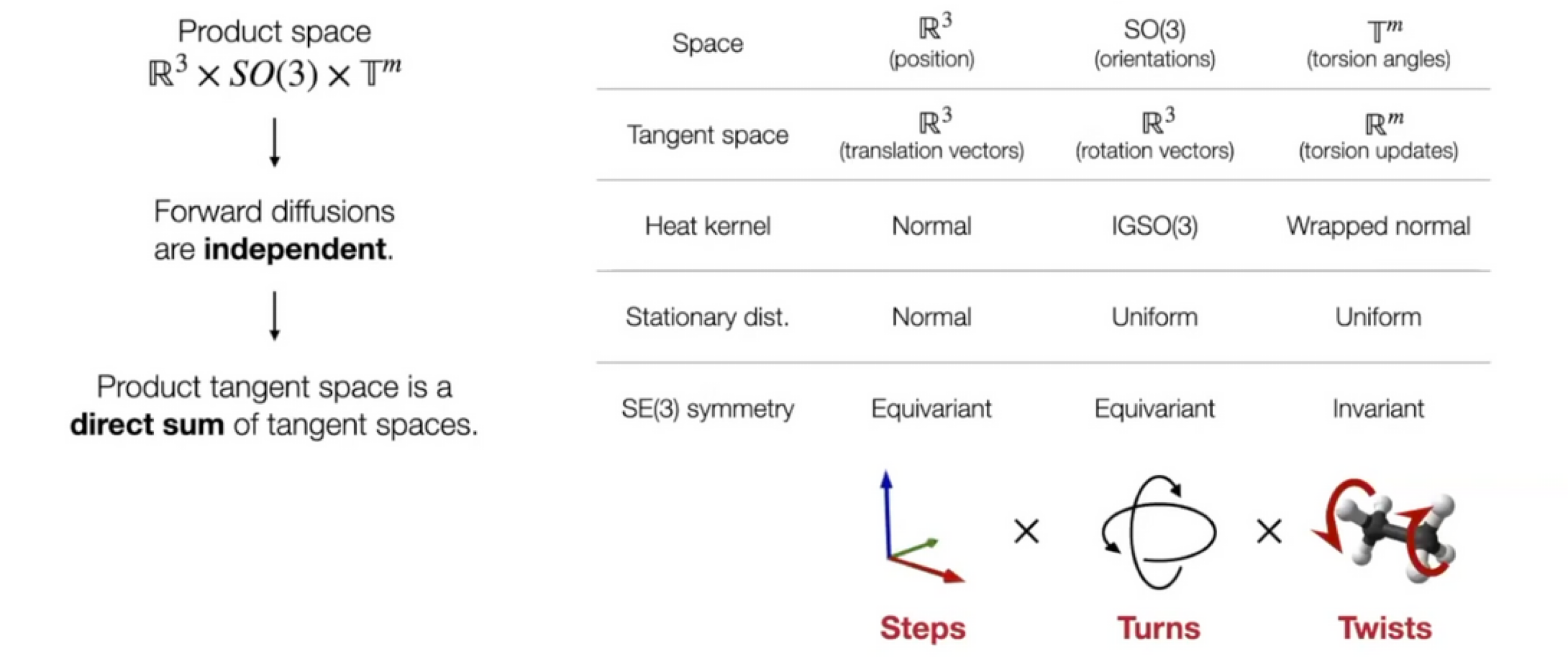
Ligand pose: $\mathbb{R}^{3n}$ ($n$: number of atoms)
-
But molecular docking needs far less degrees of freedom.
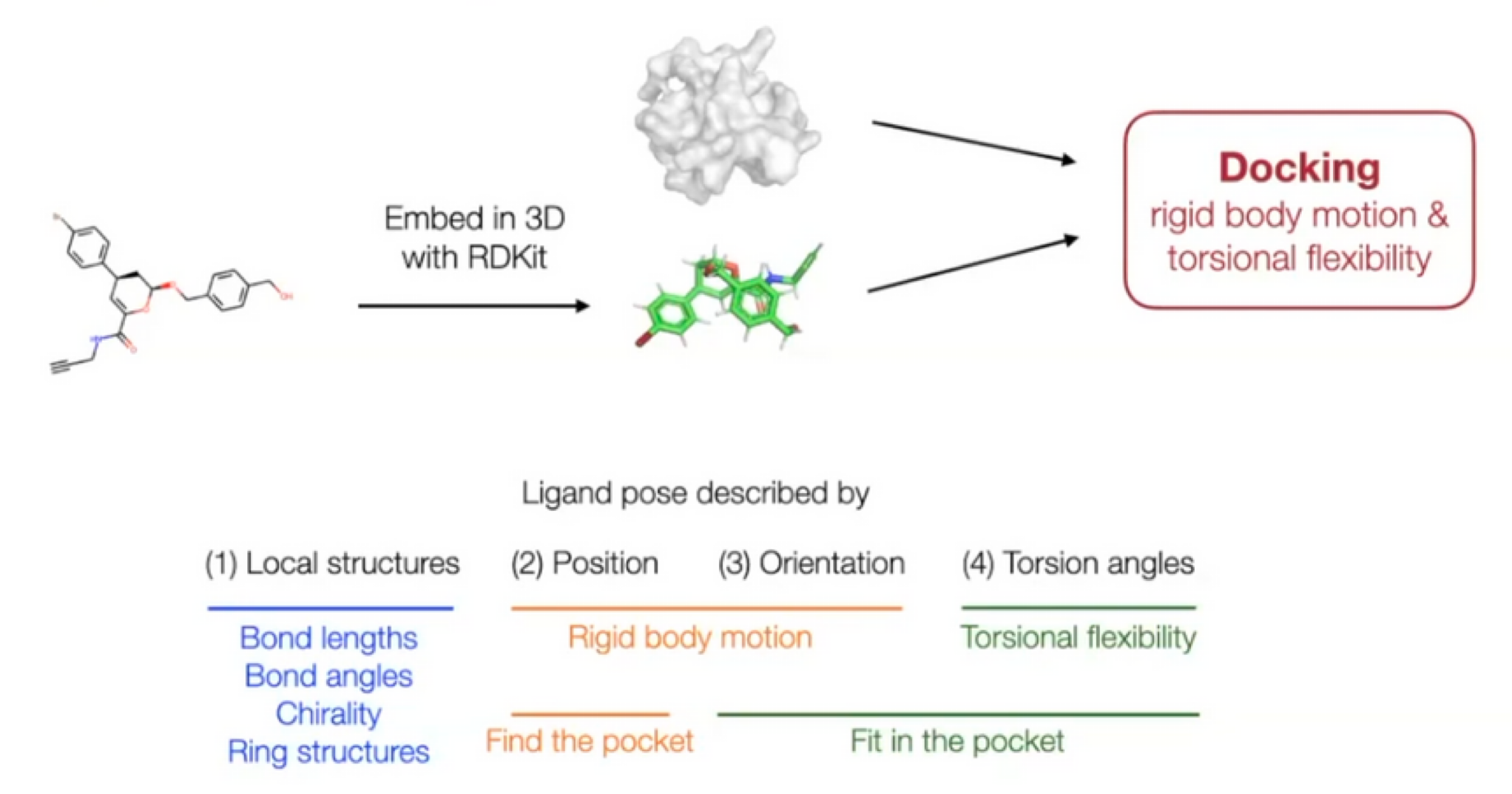
-
Reduced degree of freedom: $(m+6)$
-
Local structure: Fixed (rigid) after conformer generation with RDKit
EmbedMolecule(mol)- Bond length, angles, small rings
-
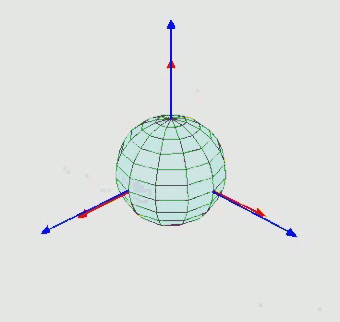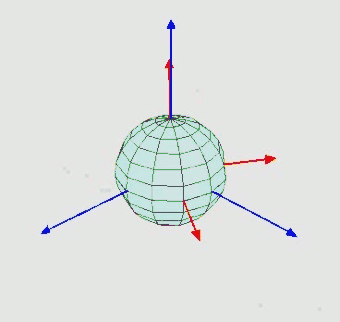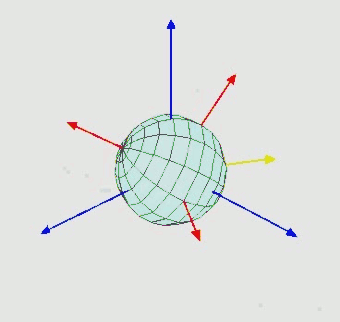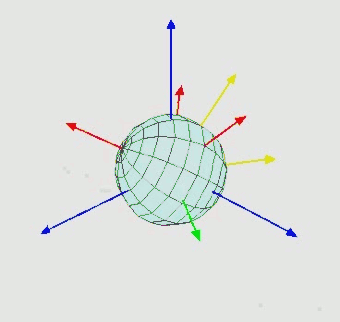
Position (translation): $\mathbb{R}^3$ - 3D vector
-
Orientation (rotation): $SO(3)$ - three Euler angle vector
-
Torsion angles: $\mathbb{T}^m$ ($m$: number of rotatable bonds)
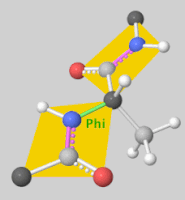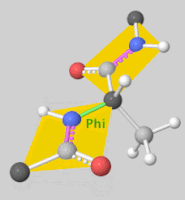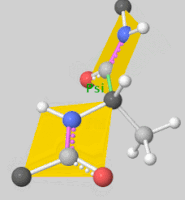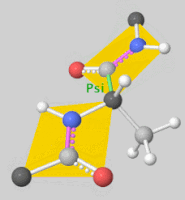
-
-
Can perform diffusion on product space $\mathbb{P}: \mathbb{R}^3 \times SO(3) \times \mathbb{T}^m$
- For a given seed conformation $\mathbf{c}$, the map $A(\cdot, \mathbf{c}): \mathbb{P} \rightarrow \mathcal{M}_\mathbf{c}$ is a bijection!
-
Confidence Model
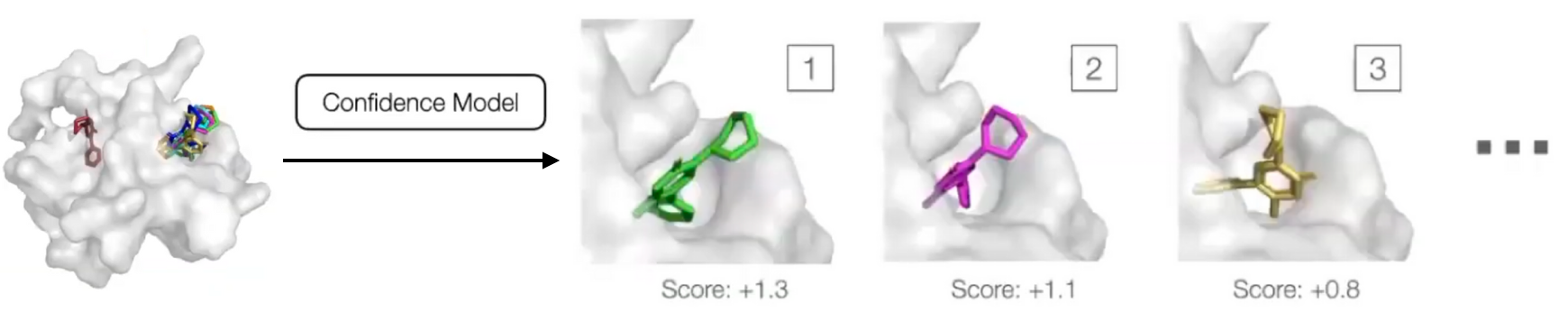
- Generative model can sample an arbitrary number of poses, but researchers are interested in one or a fixed number of them.
- Confidence predictions are very useful for downstream tasks.
- Confidence model $d(\mathbf{x}, \mathbf{y})$
- $\mathbf{x}$: pose of a ligand
- $\mathbf{y}$: target protein structure
- Samples are ranked by score and the score of the best is used as overall confidence score.
- Training & Inference
- Ran the trained diffusion model to obtain a set of candidate poses for every training example and generate binary labels: each pose has RMSD below $2 \text{Å}$ or not.
- Then the confidence model is trained with cross entropy loss to predict the binary label for each pose.
- During inference, diffusion model is run to generate $N$ poses in parallel, and passed to the confidence model that ranks them based on its confidence that they have RMSD below $2\text{Å}$.
DiffDock Workflow
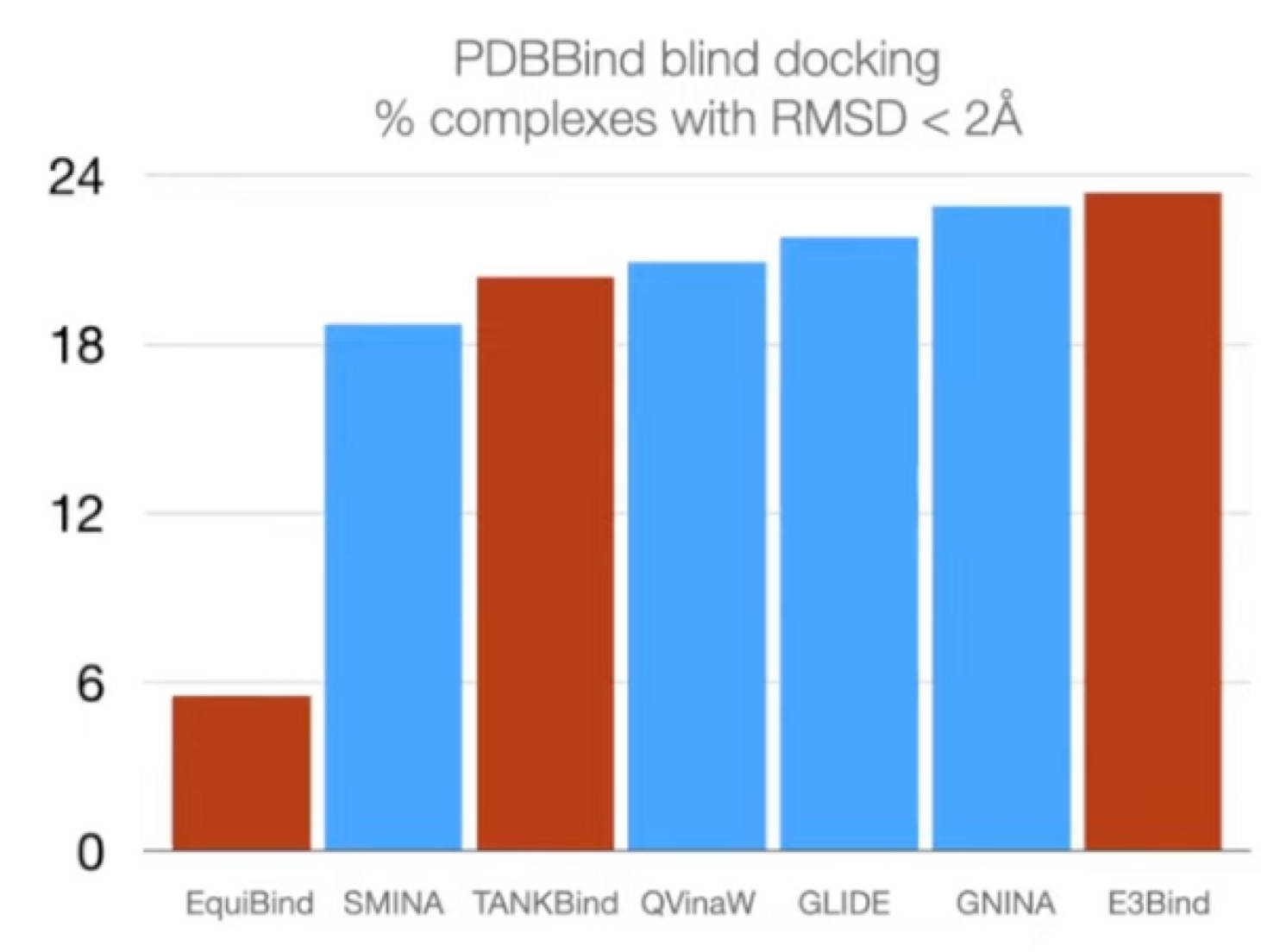
DiffDock Results
-
Standard benchmark: PDBBind
19k experimentally determined structures of small molecules + proteins
Baselines: search-based & deep learning
-
Prediction correctness
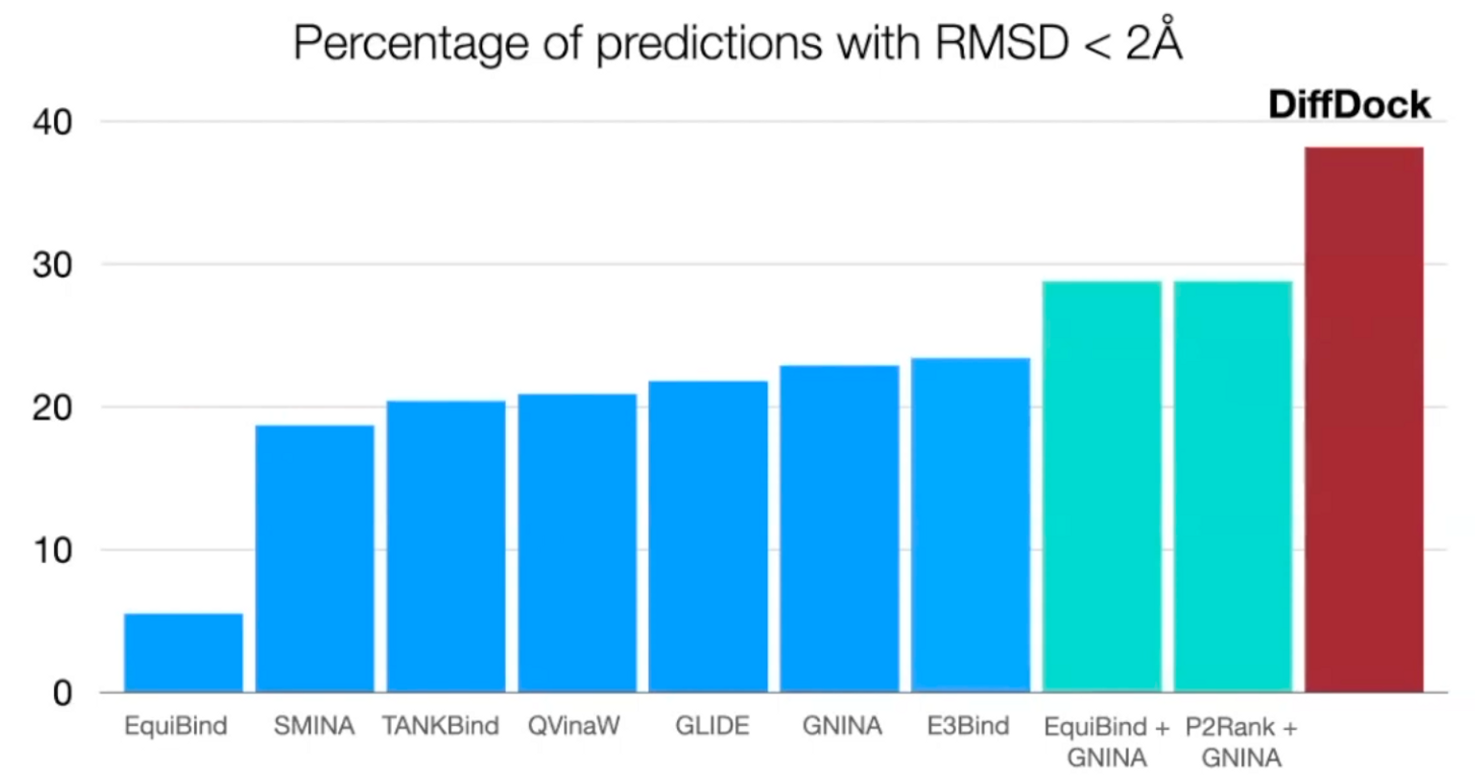
Outperform search-based, deep learning, and pocket prediction + search-based methods
-
Runtime
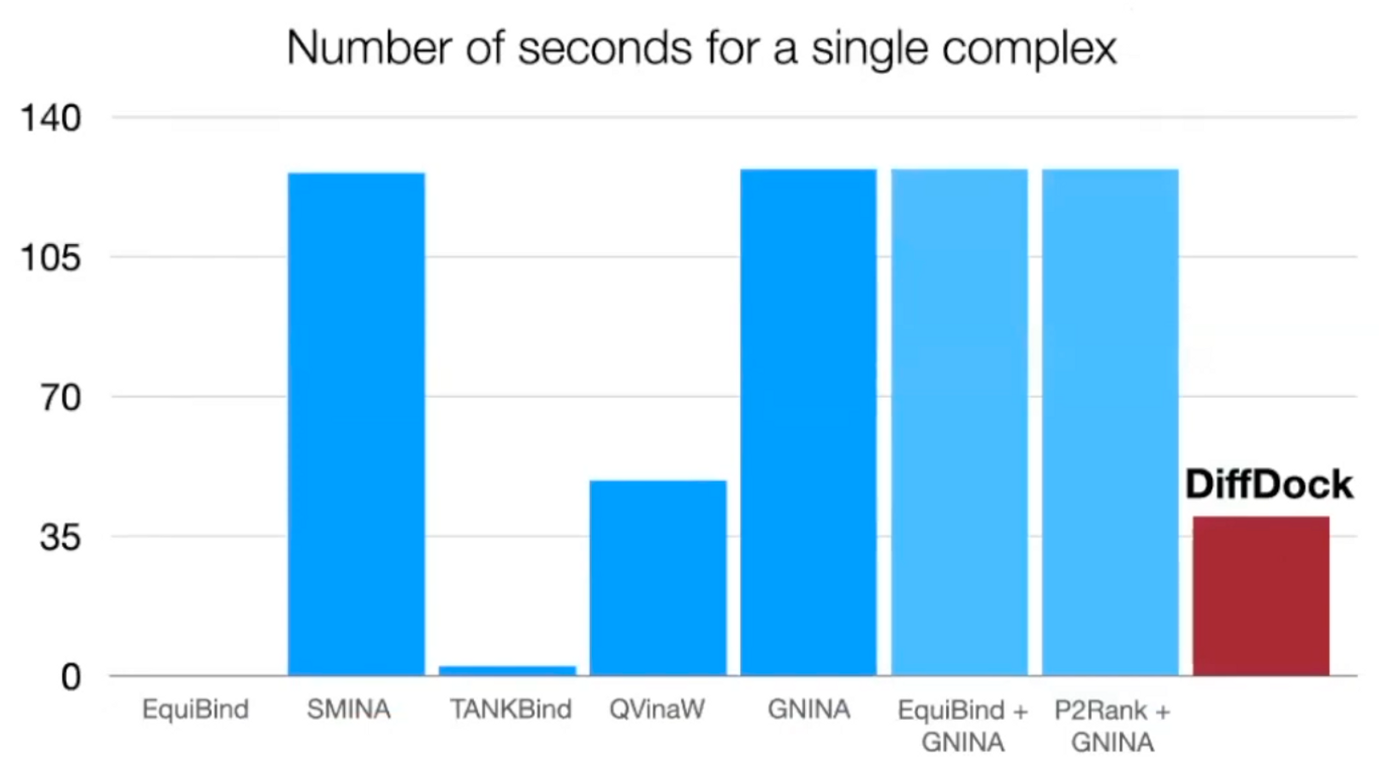
3 times faster than the most accurate baseline
-
Generalization to unseen receptors
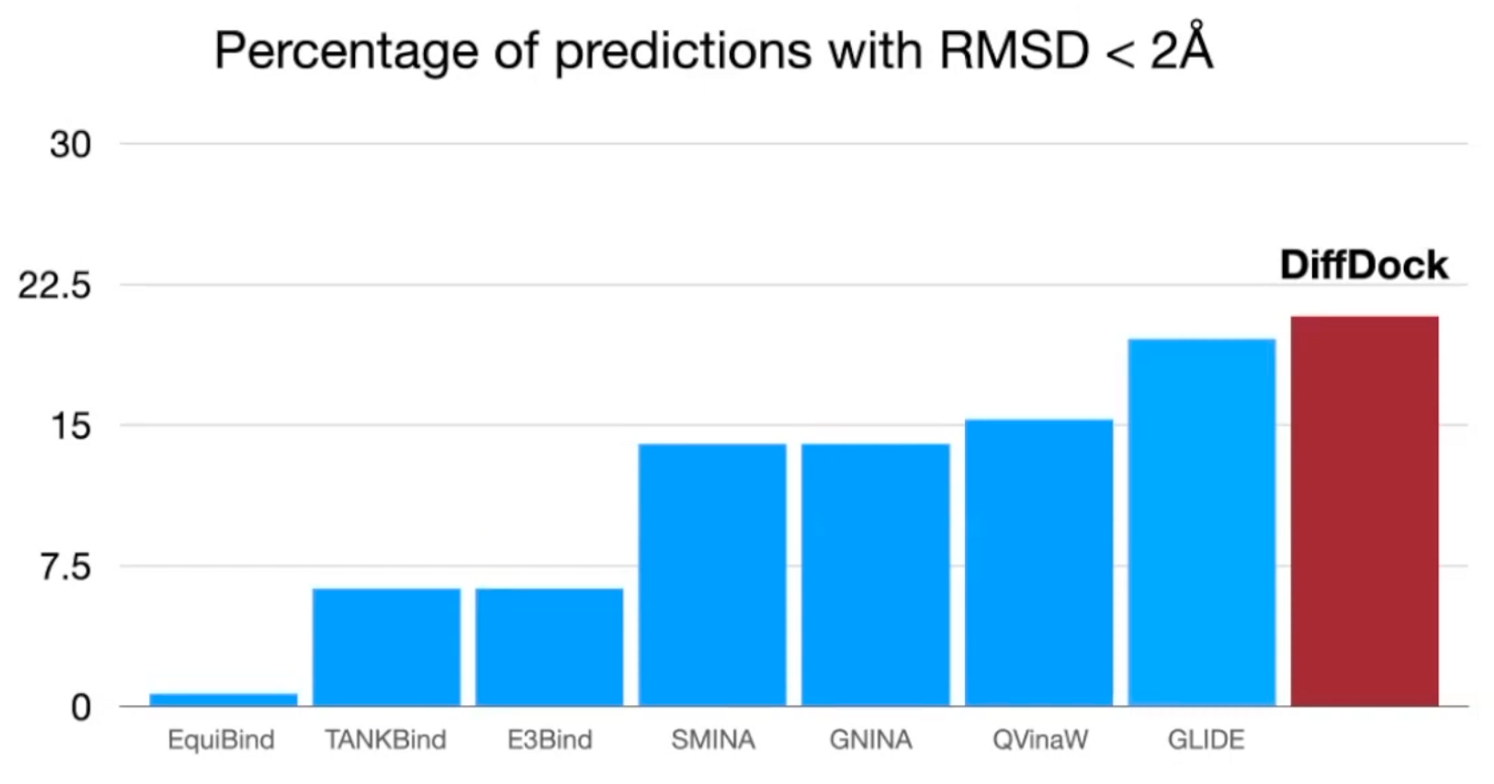
Able to generalize: outperform classical method
-
Reverse diffusion process GIF
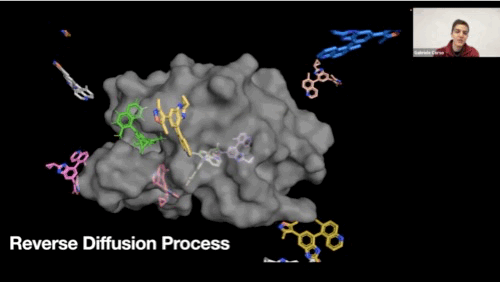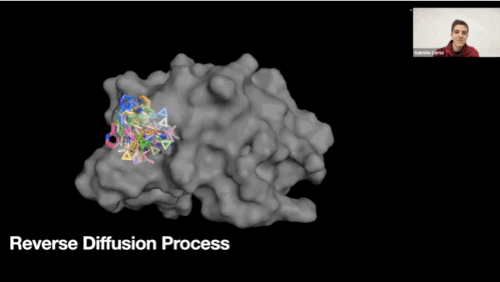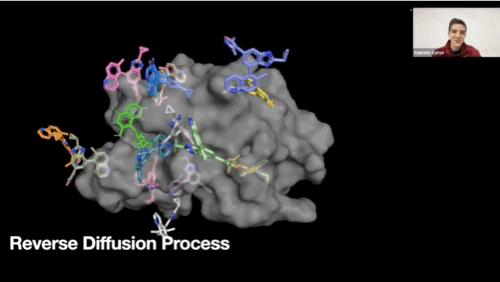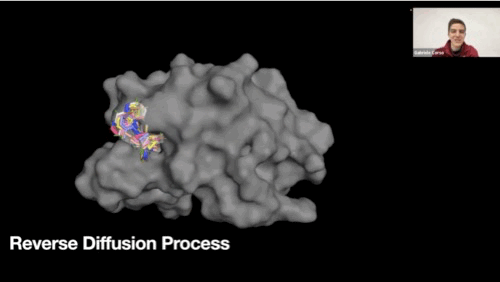
-
Confidence score quality
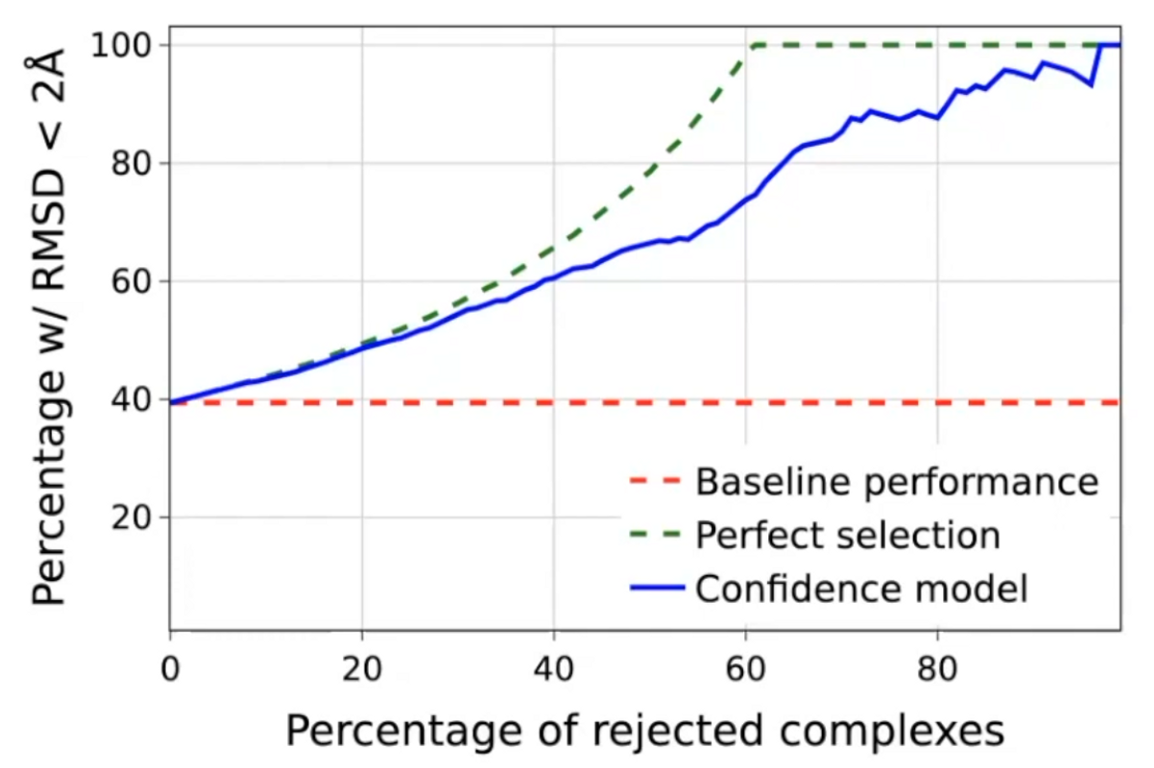
High selective accuracy: valuable information for practitioners
Personal opinions
- It is impressive that the authors formulated molecular docking as a generative problem, conditioned on protein structure.
- But it is not an end-to-end approach. And there are some discrepancy between the inputs and output of the confidence model. The input is the predicted ligand pose $\hat{\mathbf{x}}$ and protein structure $\mathbf{y}$, but the output is “whether the RMSE is below 2Å between predicted ligand pose $\hat{\mathbf{x}}$ and ground truth ligand pose $\mathbf{x}$”.
- There are quite a room to improve the performance, but it requires heavy workloads of GPUs.
- I’m skeptical about the generalizability of this model since there are almost no physics informed inductive bias in the model.